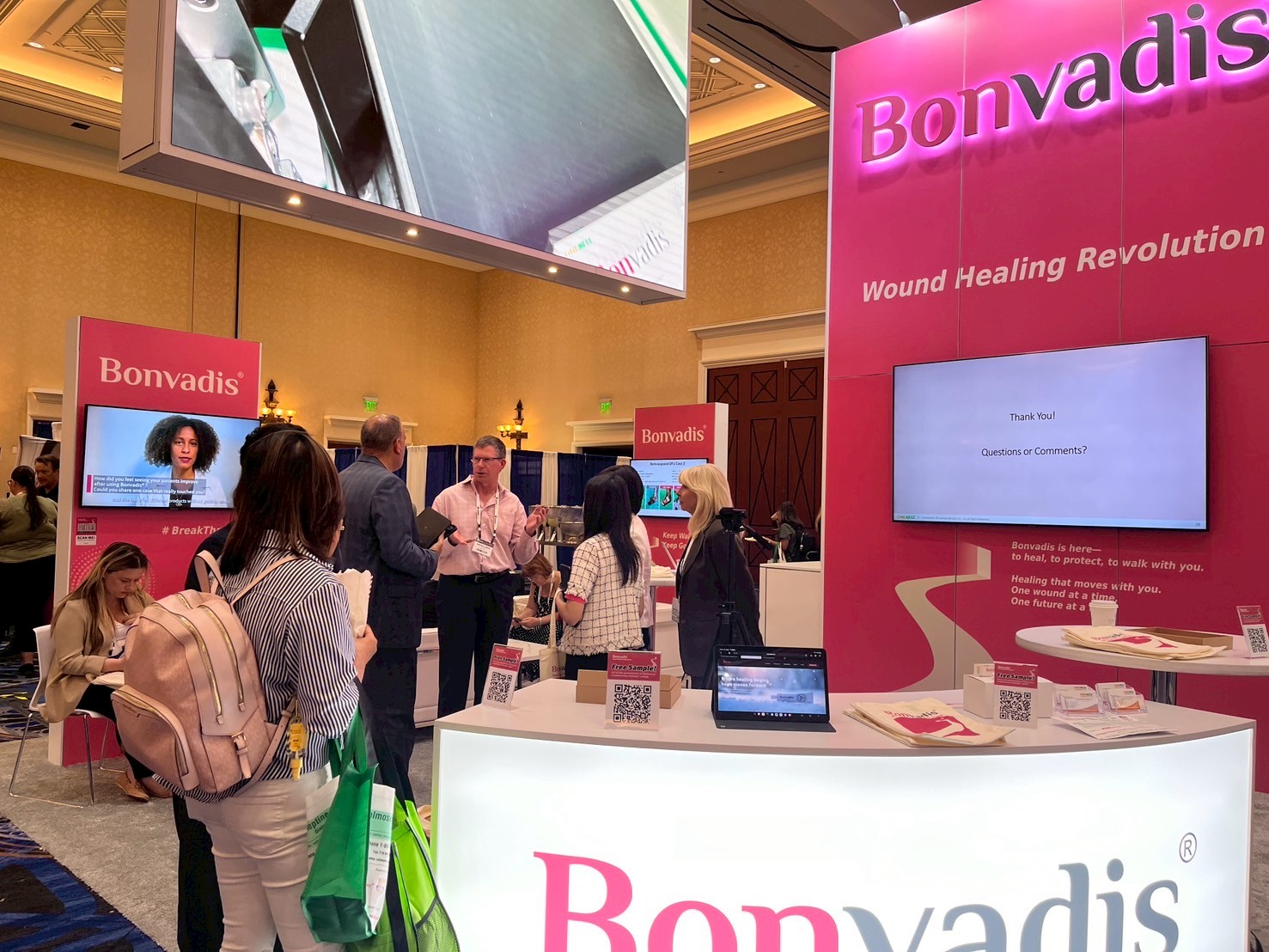
Oneness Biotech Co., Ltd. is pleased to share the successful conclusion of our participation at the Symposium on Advanced Wound Care (SAWC) Fall 2025, one of the premier global events dedicated to wound care. The conference was held from September 3–6, 2025, at Caesars Palace in Las Vegas, Nevada, USA.
At Booth #637, Oneness Biotech showcased Bonvadis®, our innovative, FDA-approved wound healing device developed to support the treatment of acute, chronic, and complex wounds. Throughout the exhibition, we had the opportunity to connect with wound care clinicians, researchers, and industry leaders, engaging in meaningful discussions about advancing patient care.
Our participation underscored Oneness Biotech’s commitment to expanding its presence in the U.S. market and to collaborating with healthcare professionals seeking effective, evidence-based solutions for wound management.
Bonvadis® continues to represent a major advancement in the treatment of chronic wounds. Backed by over 15 years of research and clinical development, its proprietary botanical-based formulation is designed to optimize the wound healing environment. With FDA approval, Bonvadis® is now positioned as a life-changing innovation for patients worldwide.
We thank all the clinicians, partners, and attendees who visited our booth and shared their insights. Your support strengthens our mission to deliver better wound care solutions to patients in need.
Oneness Biotech at SAWC Fall 2025
Booth number: 637
Date: September 3–6, 2025
Location: Caesars Palace in Las Vegas, Nevada, USA
SAWC Fall 2025 Official Website